Your Guide to the 2022 Changes to the ADA Standards of Care
The American Diabetes Association released its 2022 Standards of Care, which provides its annual update on practice guidelines. Here's what these new updates mean, including your options for first-line glucose-lowering therapies, when you should be screened for diabetes, the expanded use of diabetes care technology, and more.
The American Diabetes Association (ADA) recently released its 2022 Standards of Medical Care in Diabetes. This document is updated every year in January by the ADA’s Professional Practice Committee (PPC), which reviews recent research and consults with subject matter experts. The goal is to provide healthcare professionals, researchers, insurers, and people with diabetes and their loved ones with guidelines on the diagnosis and management of diabetes. The updates this year focus on screening, preventing complications, use of technology, and individualizing diabetes care.
“The science of diabetes care continues to evolve, and as new innovations and understandings happen, the ADA is committed to getting this information out as quickly as possible to provide the best care for people with diabetes,” said Dr. Robert Gabbay, the chief scientific and medical officer of the ADA.
While these Standards are created to guide healthcare professionals, having knowledge of these diabetes care guidelines can help people with diabetes work with their healthcare providers to advocate to get the best care possible. “We believe in empowering people with diabetes to be advocates for their own best care,” Gabbay added. “We typically see that the more people know, the better they do.”
With this in mind, here is a guide to help you understand the changes to the Standards of Care so you can become your own best advocate.
Which diabetes medications should I use for type 2 diabetes and when?
The ADA updated its recommendations for the initial medications to use to manage glucose, lipids, blood pressure, and several diabetes complications following diagnosis.
First-line medication options beyond metformin to manage glucose
The new guidelines recommend an individualized approach from the moment of diagnosis of type 2 diabetes. This means taking into account the goal to prevent complications of diabetes (such as heart or kidney disease), cost, access to care, and individual management needs. While the guidelines specify that the first medication that should be prescribed should usually be metformin in addition to comprehensive lifestyle changes (such as referral to a diabetes self-management education and support programs and medical nutritional therapy), this allows for more flexibility than the 2021 recommendation, which said that all people should be prescribed metformin.
“We know now that many of these medications that lower cardiovascular [heart] and renal [kidney] disease can be quite effective, often literally life-saving,” said Gabbay. “Metformin is still a good drug, but it should not be a deterrent to work quickly starting medications we know will be effective.”
Gabbay added that in some instances, “to reduce cardiovascular or renal [kidney] risk, these other medications are more appropriate earlier, and we hope the change in recommendations will ensure people get the medications they need earlier on.”
These updates are posted in Chapter 3 of the 2022 ADA diabetes care guidelines.
A four-pillar approach
The ADA also modified its recommendations on how to manage diabetes-related complications, including heart failure, chronic kidney disease (CKD), obesity, retinopathy, and more.
The figure below represents a new four-pronged approach for people with diabetes to reduce the risk of developing these complications.
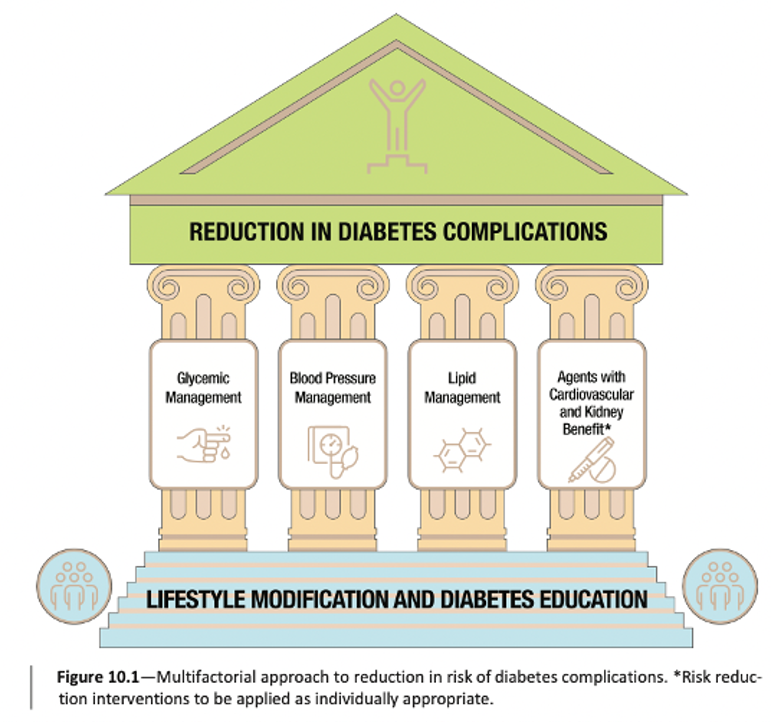
As you can see, this approach includes four pillars: glucose management, blood pressure management, lipid management, and using glucose-lowering medications that have been shown to have heart or kidney benefits, all atop a foundation of lifestyle modification and diabetes self-management education and support. This marks a key transition to a more comprehensive approach to diabetes management.
For more details on this approach, see Chapter 10 of the 2022 ADA diabetes care guidelines.
SGLT-2 inhibitors are now recommended to treat heart failure and can be started at the time of diagnosis
SGLT-2 inhibitors were previously recommended only to treat one type of heart failure (heart failure with reduced ejection fraction, or HFrEF), but the ADA now encourages this category of medications for treating and preventing other types of heart failure, based on exciting clinical trial results from this past year.
These updates are posted in Chapter 10 of the 2022 ADA diabetes care guidelines.
Finerenone can be used to treat CKD when SGLT-2 inhibitors are not well-tolerated
The updated guidelines now suggest that certain individuals who have stage 4 CKD to take SGLT-2 inhibitors to preserve kidney function. In the past, ADA recommended that after progressing to stage 4 kidney disease, people should stop using SGLT-2s, as the risk for additional kidney damage actually increased at advanced stages. The updated guidelines changed this threshold, suggesting that more people in advanced stages of CKD can now safely use an SLGT-2 inhibitor.
Some people, however, may not respond well to treatment with an SGLT-2 inhibitor. In this case, finerenone (Kerendia), a recently approved non-steroidal MRA drug, can alternatively be used to improve both kidney and heart outcomes.
These updates are posted in Chapter 11 of the 2022 ADA diabetes care guidelines.
Combination therapy “may be considered” for people with established heart or kidney disease
A combination of medications using two or more different types of drugs, has been effective in helping people manage their diabetes. The ADA now recommends that people with type 2 diabetes who take insulin combine insulin with a GLP-1 receptor agonist (such as Rybelsus, Ozempic, Bydureon, Trulicity, Victoza, etc.) if additional glucose lowering is needed, as opposed to only increasing insulin dosing.
Past ADA guidelines recommended using an SGLT-2 inhibitor or a GLP-1 receptor agonist for heart or kidney disease. This year, however, recommendations suggest that a combination of the two should be considered to lower risk even more. In addition, instead of adding the drugs one by one, it may be best to start with a combination of the two depending on the individual’s situation.
These updates are posted in Chapter 10 of the 2022 ADA diabetes care guidelines.
Overweight or obesity therapy recommendations now include Wegovy, emphasize importance of food quality over quantity
The new guidelines also now recommend Wegovy (semaglutide 2.4mg) as an effective therapy for weight management for people with type 2 diabetes. For those with type 2 who take insulin, however, using Wegovy at the same time may increase the risk for hypoglycemia. The drug can still be an effective method to achieve some weight loss, but people should get educated on the signs, symptoms, and risk of hypoglycemia before starting this medication.
The guidelines also recommend managing glucose through more than just carbohydrate-counting. Regardless of the amount of carbohydrate in the meal plan, people should focus on eating high-quality and nutrient-dense carbohydrate sources that are high in fiber. Both children and adults should limit the amount of refined or processed carbs they eat that include added sugars, fat, and salt and instead focus on getting their carbs from vegetables, legumes, fruits, dairy (milk and yogurt), and whole grains.
These updates are posted in Chapter 8 of the 2022 ADA diabetes care guidelines.
Which diabetes technologies should I use?
The ADA expanded recommendations for continuous glucose monitor (CGM) and Time in Range use in adults and for CGM and automated insulin delivery (AID) use in children. The guidelines also include using diabetes technology in hospital settings.
14-day CGM assessment of Time in Range (TIR) and glucose management indicator (GMI) recommended for glucose management
A1C has long been considered the gold standard diabetes metric, but TIR and other CGM metrics have been gradually incorporated into the Standards of Care as complementary measures to A1C. The ADA now recommends evaluating glucose management using a 14-day assessment from CGM, Time in Range and GMI, which can be used to gain insight into the ups and downs of glucose over time. The Standards of Care explains that Time in Range, Time below Range, and Time above Range are all useful tools to help healthcare professionals with medical decision-making. These metrics can also help people with diabetes in their day-to-day diabetes management.
The Standards also highlighted the importance of evaluating a person’s risk of hypoglycemia and described low blood sugar, if it occurs, as an urgent issue. While in past years the Standards encouraged healthcare providers to look at how often a person experiences or is at risk for hypoglycemia, the recommendations now include hypoglycemia education and adjustment of therapy.
“In this year’s Standards, we expanded on our recommendations for what to do with that information (i.e., Time in Range, Time below Range, etc),” said Gabbay. “We especially wanted to bring greater attention to Time below Range. The conversation does not always have to result in a therapeutic change, but alerting people to focus on their Time below Range can help people assess and act quickly.”
These updates are posted in Chapter 6 of the 2022 ADA diabetes care guidelines.
CGM recommended for all adults who take insulin, including basal-only
Last year’s ADA guidelines recommended that people who take rapid-acting insulin (such as Novolog, Humalog, Fiasp, and Lyumjev, among others) should use a CGM. This year, the ADA expanded this recommendation to include people who take only long-acting insulin (often referred to as basal insulin). Research on CGM use in people with type 2 diabetes indicates that the devices can help those on basal-only insulin improve their day-to-day glucose management. The 2022 guidelines also recommend CGM for all children with type 1 and type 2 diabetes who use rapid-acting insulin.
“Studies over the last year have made clear that if individuals are on insulin, no matter who or what age, they can benefit from CGM use,” said Gabbay. “Some evidence even suggests that CGM could help people who are not on insulin, but this evidence base will need some strengthening going forward.”
AID and CGM recommended for children with diabetes
The 2022 guidelines expand recommendations for diabetes technology use among all children who use rapid-acting insulin. For children with type 1 diabetes, the ADA also recommends automated insulin delivery (AID) systems. You can learn more about these AID systems at our resource page here.
CGM and AID systems have proven to improve health outcomes in children, and these insulin delivery technologies allow families to track their children’s health data remotely as well as manage instances of hypoglycemia and hyperglycemia, among other things.
Diabetes technology use in hospitals
Before the COVID-19 pandemic, people with diabetes were not always allowed to use their CGMs, insulin pumps, and AID systems while hospitalized, but the guidelines for using these devices have become more flexible in recent years to prevent the spread of COVID-19, though they vary from institution to institution. It’s important for people with diabetes and their loved ones to continue to advocate for the use of these technologies in hospitals.
The ADA’s 2022 guidelines discuss in-hospital technology use for all people who are able to safely use their devices in the hospital setting.
How has diabetes care changed?
The ADA also lowered the age to screen for prediabetes and type 2 diabetes to 35 years, encouraged healthcare providers to individualize care, and recommended COVID-19 vaccines for all adults with diabetes.
Prediabetes and type 2 screening should start at age 35
The ADA now recommends that adults who do not have diabetes symptoms should be screened for prediabetes and type 2 diabetes starting at age 35. This change comes after the US Preventive Services Task Force (USPSTF) lowered its recommended screening age from 45 to 35 years in August, 2021. Of the estimated 34 million US adults with diabetes in 2018, about one in five (21%) was undiagnosed. The CDC estimates that 88 million people in the US have prediabetes and that most of them don’t know it and have not been made aware by their healthcare providers.
“The prevalence of diabetes is rising; there are many people with type 2 diabetes who are undiagnosed, and it is occurring at younger and younger ages,” said Gabbay. “I saw a patient who was diagnosed with diabetes after seeing his eye doctor, who determined that he already had significant retinopathy, eventually losing vision in one eye. He presumably had diabetes for a number of years but never knew it. This should not have to happen, which is why this change is important.”
The guidelines also added new screening recommendations for pregnant women and those planning a pregnancy. Those at risk for diabetes who are planning to become pregnant should be screened before conception or, if not screened before conception, before they are 15 weeks pregnant. The standards also urge healthcare professionals to consider screening all those who are currently pregnant or planning to become pregnant regardless of diabetes risk.
“Many women with pre-existing [type 2] diabetes become pregnant but don’t know they have diabetes. That’s why we now recommend screening within this time frame [to identify pre-existing diabetes] in addition to typical screenings in the third trimester.”
These updates are posted in Chapter 2 of the 2022 ADA diabetes care guidelines.
Healthcare providers are encouraged to individualize diabetes care
While the Standards focus on general protocols for treating all people with diabetes, they stress the importance of individualized care based on people’s unique needs.
Putting the person, rather than their diabetes, at the center of healthcare can help improve person-provider relationships as well as physical and mental health outcomes, and it can reduce the stigma people with diabetes experience in healthcare.
COVID-19 vaccines recommended for all adults and some children
Last year’s guidelines were released before the COVID-19 vaccines were available. Now that the Pfizer, Moderna, and Janssen (Johnson & Johnson) vaccines have been approved by the FDA, the 2022 guidelines urge all adults, including those with diabetes, to get fully vaccinated. All children who are eligible should also be fully vaccinated.
COVID-19 has disproportionately impacted people with diabetes who are at a higher risk for serious COVID-related health complications. The vaccines can help prevent these health complications as the virus continues to spread in the US. The ADA notes that the COVID-19 vaccine will likely become a routine part of annual preventive healthcare for people with diabetes in addition to an annual flu shot.